Wu ZS. et al., 2025: Antibacterial and therapeutic effects of low energy shock waves on uropathogenic E. coli investigated by in vitro and in vivo cystitis rat model.
Zong-Sheng Wu 1 2, Cheng-Yen Kao 3 4, Hung-Jen Wang 1 2, Wei-Chia Lee 1 2, Hou Lun Luo 1 2, Chao-Cheng Huang 5, Yao-Chi Chuang 6 7 8
1Department of Urology, Kaohsiung Chang Gung Memorial Hospital and Chang Gung University College of Medicine, Kaohsiung, 833, Taiwan.
2Center for Shockwave Medicine and Tissue Engineering, Kaohsiung Chang Gung Memorial Hospital and Chang Gung University College of Medicine, Kaohsiung, 833, Taiwan.
3Institute of Microbiology and Immunology, College of Life Sciences, National Yang Ming Chiao Tung University, Hsinchu, Taiwan.
4Health Innovation Center, National Yang Ming Chiao Tung University, Hsinchu, Taiwan.
5Department of Pathology, Kaohsiung Chang Gung Memorial Hospital, Chang Gung University College of Medicine, Kaohsiung, Taiwan.
6Department of Urology, Kaohsiung Chang Gung Memorial Hospital and Chang Gung University College of Medicine, Kaohsiung, 833, Taiwan.
7Center for Shockwave Medicine and Tissue Engineering, Kaohsiung Chang Gung Memorial Hospital and Chang Gung University College of Medicine, Kaohsiung, 833, Taiwan.
8School of Medicine, College of Medicine, National Sun Yat-sen University, Kaohsiung, 833, Taiwan
Abstract
Aims: Low-energy shock waves (LESWs) are known to alter cell-membrane permeability. This study aimed to investigate the effect of LESWs on Escherichia coli and E. coli-induced cystitis in rats.
Main methods: Standardized suspensions of E. coli ATCC25922 were treated with or without LESWs (100 or 300 pulses; 0.12 mJ/mm2; 2 pulses/s) followed by bacterial counting, an antibiotic sensitivity test, and gene ontology analysis and gene-set enrichment analysis. Intravesical administration of saline or E. coli (0.5 mL with 108 CFU/mL) for 30 min was performed in female Sprague-Dawley rats. The rats were treated with or without LESWs (300 pulses; 0.12 mJ/mm2; 2 pulses/s) on days 4 and 5. The changes in inflammatory reactions, uroplakin IIIa staining, and correlation with urodynamic findings were assessed on day 8.
Key findings: LESW treatment induced a decrease in CFU and the autoaggregation rate and increased the inhibition zone sizes in a cefazolin-sensitivity study. These changes were associated with gene expression in regulation of cellular membrane components, biofilm formation, and the ATP-binding cassette transporter pathway. E. coli induced bladder hyperactivity and an inflammatory reaction as well as decreased uroplakin IIIa staining; these effects were partially reversed by LESW treatment.
Significance: The LESW antibacterial effect occurs by altering bacterial cell-membrane gene expression, enhancing antibiotic sensitivity, and inhibiting bladder inflammatory reaction and overactivity. These findings support the potential benefits of LESWs for treatment of recurrent or refractory bacterial cystitis.
Int Urol Nephrol. 2025 Jan;57(1):49-61. doi: 10.1007/s11255-024-04173-8. Epub 2024 Jul 30. PMID: 39078466


Comments 1
This very interesting article focuses on the antibacterial effects and therapeutic potential of LESWs on E. coli both in laboratory settings and in a rat model of cystitis.
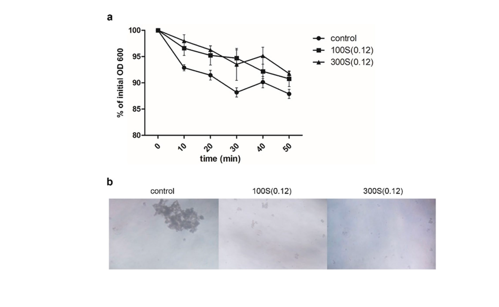
Methods: The authors used first in-vitro studies followed by in-vivo studies using the rat model.
In Vitro Experiments: E. coli ATCC25922 cultures were exposed to LESWs at 0.12 mJ/mm², delivering either 100 or 300 pulses at a frequency of 2 pulses per second using the SD-1-device od Storz-Medical, Taegerwilen, Switzerland). Post-treatment analyses included bacterial colony counts, antibiotic sensitivity tests, and gene expression studies focusing on membrane components and biofilm formation.
In Vivo Experiments: 18 Female Sprague–Dawley rats were intravesically instilled with E. coli to induce cystitis. LESW treatments (300 pulses; 0.12 mJ/mm²; 2 pulses/s) were administered on days 4 and 5 post-infection. Evaluations on day 8 included assessments of bladder inflammation, uroplakin IIIa expression, and urodynamic measurements.
Key Findings
Antibacterial Effects: LESW treatment significantly reduced bacterial colony-forming units (CFUs) and autoaggregation rates.
Enhanced sensitivity of E. coli to the antibiotic cefazolin was observed, indicated by increased inhibition zone sizes. Gene expression analyses revealed alterations in pathways related to membrane components, biofilm formation, and ATP-binding cassette transporters
Therapeutic Effects in Rats: LESW-treated rats exhibited reduced bladder inflammation and improved uroplakin IIIa expression. Urodynamic studies indicated amelioration of bladder overactivity induced by E. coli infection.
Discussion
The study suggests that LESWs can disrupt E. coli cell membranes, enhancing antibiotic susceptibility and reducing virulence factors like biofilm formation. In the rat model, LESW therapy alleviated bladder inflammation and dysfunction associated with bacterial cystitis.
Limitations: There are basically two limitations.
- The Translational Gap:
While results in rats are promising, human studies are necessary to confirm efficacy and safety.
- Mechanistic Insights:
Further research is needed to elucidate the precise molecular mechanisms by which LESWs exert antibacterial effects.
Underlying mechanism: Extracorporeal Shock Wave Therapy (ESWT) is classically understood to work through mechanotransduction — converting mechanical stimuli (the shockwaves) into biochemical and cellular responses. In orthopedic, urological, and dermatological applications, it promotes angiogenesis, reduces inflammation, modulates pain via nerve effects, and enhances tissue regeneration. But here, the study shows a direct antibacterial effect on E. coli — disrupting membranes, reducing biofilm formation, and increasing antibiotic susceptibility — which doesn’t fit the traditional mechanotransduction pathway, at least not entirely.
A few possibilities, based on what we know from shockwave physics and cell biology might be relevant:
Physical Disruption of Bacterial Cell Membranes: At certain even low energy levels (like the 0.12 mJ/mm² used here), shockwaves can induce shear forces and rapid pressure changes that physically compromise bacterial membranes — increasing permeability or causing lysis.
Biofilm Disruption:Biofilms are notoriously difficult to penetrate with antibiotics. Shockwaves might mechanically disrupt the extracellular matrix of biofilms, exposing bacteria to antibiotics or host immune defenses.
Interference with Bacterial Stress Responses: The gene expression changes noted (membrane components, ABC transporters, biofilm formation genes) suggest that LESW is triggering stress responses or impairing the bacteria’s ability to manage mechanical trauma.
Enhanced Antibiotic Penetration: By disrupting membrane integrity, shockwaves could facilitate better antibiotic diffusion into bacterial cells, as suggested by the increased cefazolin sensitivity.
Is this Mechanotransduction?
In eukaryotic tissues — yes, mechanotransduction is key to ESWT’s effects.
In bacteria — not so much. Bacteria lack the same mechanotransduction pathways as mammalian cells. Here it’s likely direct physical and membrane-level effects, plus possible interference with mechanical-sensitive bacterial stress pathways. So it’s not antibiotic per se, but it’s modulating bacterial viability and antibiotic susceptibility via non-chemical, physical means.
Conclusion:
If reproducible, this may open the door for:
• Antibiotic-sparing adjunctive therapies for UTIs (and maybe other infections)
• Biofilm-disrupting treatments, particularly for catheter-associated infections or chronic prostatitis cases with bacterial components
• Combining shockwave therapy with low-dose antibiotics to overcome resistance
Jens Rassweiler